Difference between revisions of "Safety-enhanced design (MU3))"
From OpenEMR Project Wiki
Bradymiller (talk | contribs) |
Bradymiller (talk | contribs) |
||
| Line 51: | Line 51: | ||
= Usability testing scenario = | = Usability testing scenario = | ||
:* Ed Smith is a patient in the user's clinic. In this scenario, the user will 'update Prostate Cancer Screening rule to explain the rule's usage of patient's biologic patient's sex.', 'provide feedback on incorrect | :* Ed Smith is a patient in the user's clinic. In this scenario, the user will 'update Prostate Cancer Screening rule to explain the rule's usage of patient's biologic patient's sex.', 'provide rule feedback on incorrect application of Pap Smear rule for Ed Smith due to incorrect biological sex.', and 'view and update 3rd party Decision Intervention Support source attribute information'. | ||
:* The following is the testing guide that will be used during testing by the users: [[Usability Testing Guide]] | :* The following is the testing guide that will be used during testing by the users: [[Usability Testing Guide]] | ||
Revision as of 06:32, 27 October 2024
Overview
- The first usability testing was completed by the 2022 Cohort of the Professional Certificate in Health Information Technology at Columbia University for the a1, a2, a5, a9, a14 certification criteria.
- An 2024 addendum to the usability study is being conducted by OpenEMR for the b11 certification criteria.
Usability Test Report
UCD Process
Criteria that tested
- a1: Computerized provider order entry (CPOE) – medications (2022 task 2) (completed in 2022 and hidden below)
- a2: CPOE – laboratory (2022 task 4) (completed in 2022 and hidden below)
- a5: Demographics (2022 task 1) (completed in 2022 and hidden below)
- a9: Clinical decision support (2022 task 1) (completed in 2022 and hidden below)
- a14: Implantable device list (2022 task 3) (completed in 2022 and hidden below)
- b11: Decision Intervention Support 2024 addendum (2024 task 1,2,3)
Research
- Actual rule:
- Example reports:
- https://content-main.ul.com/sites/g/files/qbfpbp306/files/2020-08/BlueEHR-2.0-SED-Report.pdf
- https://slicompliance.com/wp-content/uploads/2022/01/Prime-Clinical-EHR-Usability-Test-Report.pdf
- https://slicompliance.com/wp-content/uploads/2022/01/FreeChiro_18_SED_Report.pdf
- https://slicompliance.com/wp-content/uploads/2021/12/Strateq_EHR_UsabilityReport_08Dec2021.pdf
Usability official testing demos
- These are duplicate official testing demos that contain 1 patient (Ed Smith). Official testing demos completely reset daily at around 1:00 am pacific time; when official testing demos completely reset, they are rebuilt on top of most recent codebase. Official testing demos use private credentials (ie. public will not be able to access them) that are only known to the official testers.
Usability training demos
- The point of these training demos is to allow training by multiple users, which pick Ed Smith patient to run through the below training guides on. Training demos completely reset daily at around 1:00 am pacific time; when training demos completely reset, they are rebuilt on top of most recent codebase. Training demos use public credentials, which are username:admin password:Administrator1!
Usability testing scenario
- Ed Smith is a patient in the user's clinic. In this scenario, the user will 'update Prostate Cancer Screening rule to explain the rule's usage of patient's biologic patient's sex.', 'provide rule feedback on incorrect application of Pap Smear rule for Ed Smith due to incorrect biological sex.', and 'view and update 3rd party Decision Intervention Support source attribute information'.
- The following is the testing guide that will be used during testing by the users: Usability Testing Guide
- The task material below is for user training.
Task 1: View and update Decision Intervention Support source attribute information.
- Test:
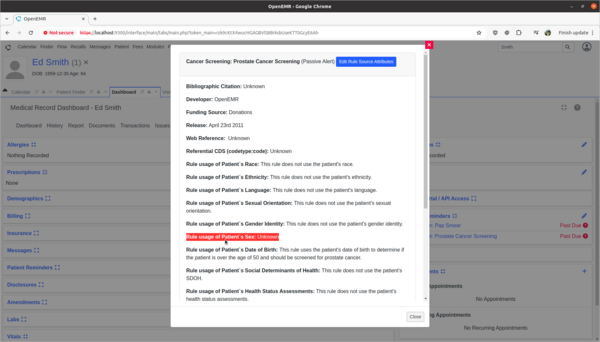
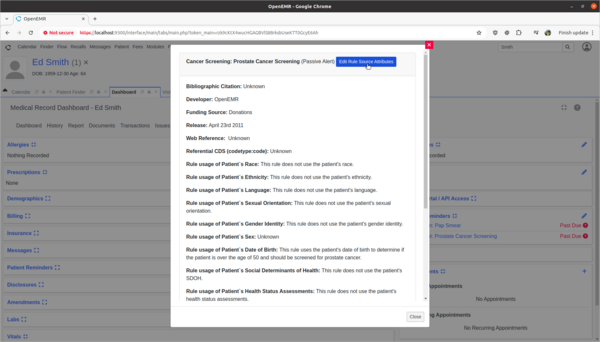
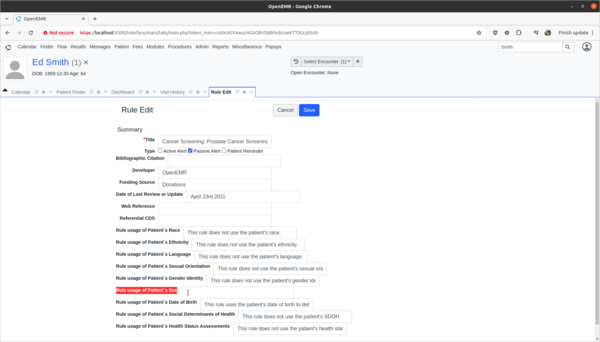
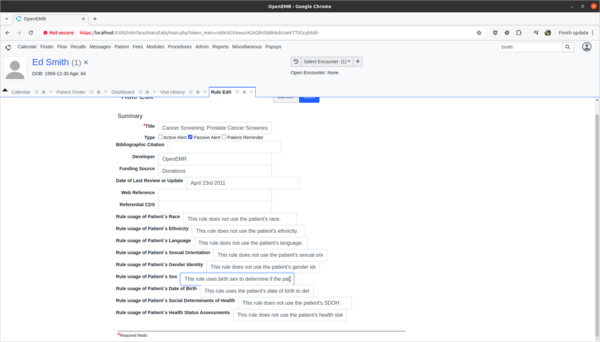
- Update Prostate Cancer Screening rule to explain the rule's usage of patient's biologic patient's sex.
- Scenario:
- The OpenEMR internal CDR is missing source attribute information on how it's Prostate Cancer Screening rule is using the patient's biological birth sex information in its rule calculations. I need to update the source attribute information for this rule to explain that the sex data point is used to determine if the patient is male and should be screened for prostate cancer. This will help clinicians make informed decisions as to the efficacy and safety of the rule.
- Specific Testing steps:
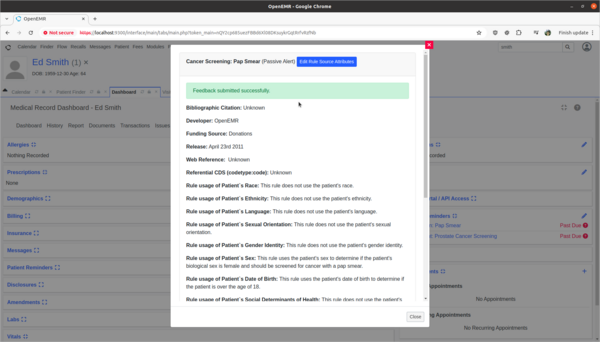
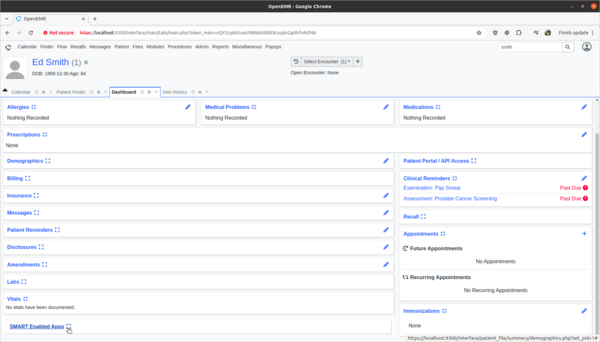
Task 2: Provide feedback on incorrect Decision Intervention Support rule usage.
- Test:
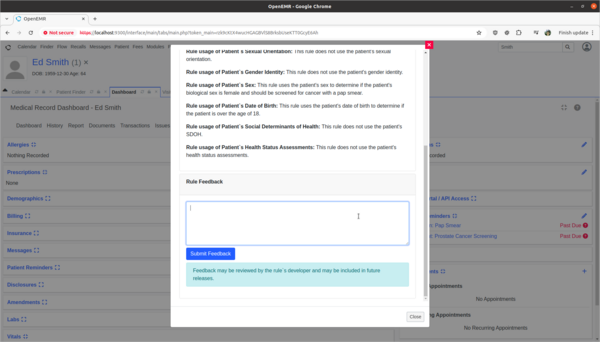
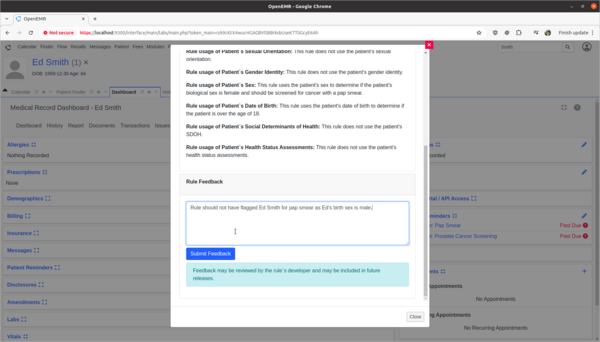
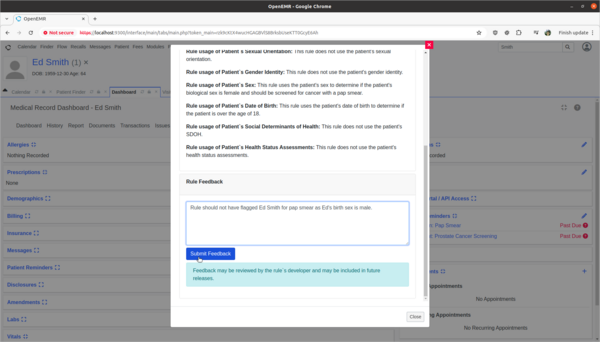
- Provide rule feedback on incorrect application of Pap Smear rule for 'Ed Smith' due to incorrect biological sex.
- Scenario:
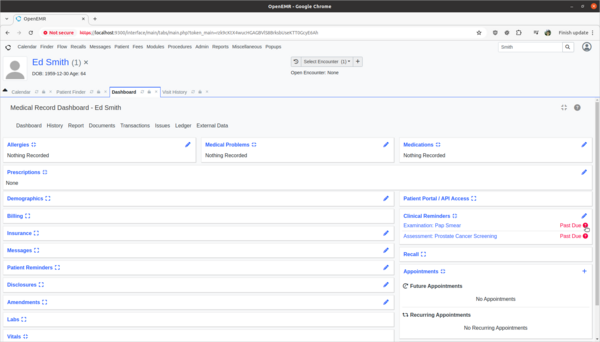
- 'Ed Smith' in the Clinical Decisions section of his patient dashboard is showing that he needs a pap smear exam. However, Ed's biological birth sex is male and he has no cervix. As a clinician I need to provide feedback to the CDR designer of this rule that it is improperly being applied to Ed and the rule needs to be fixed.
- Specific Testing steps:
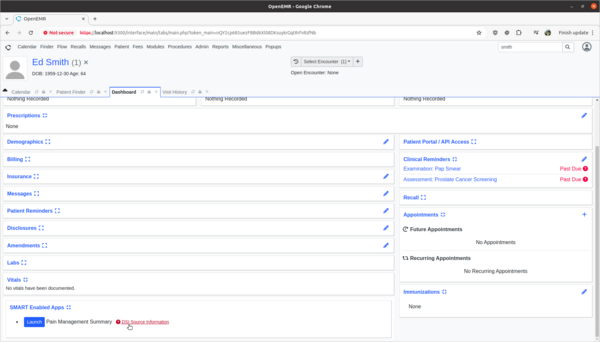
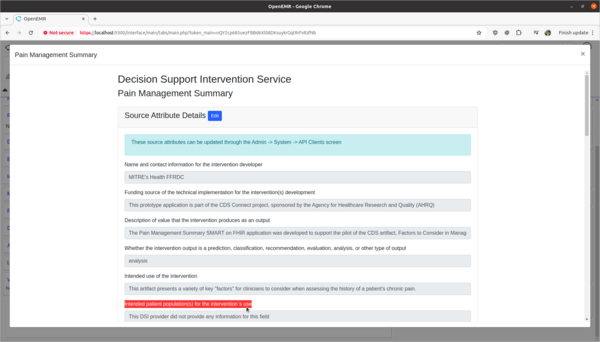
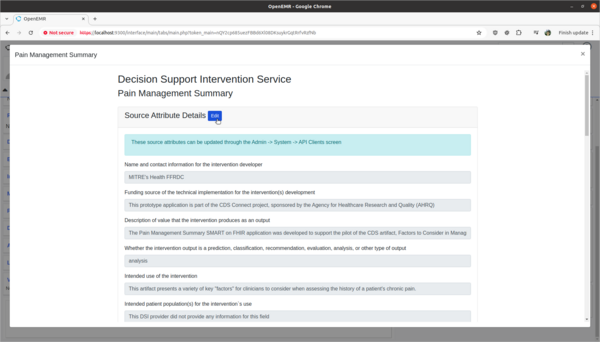
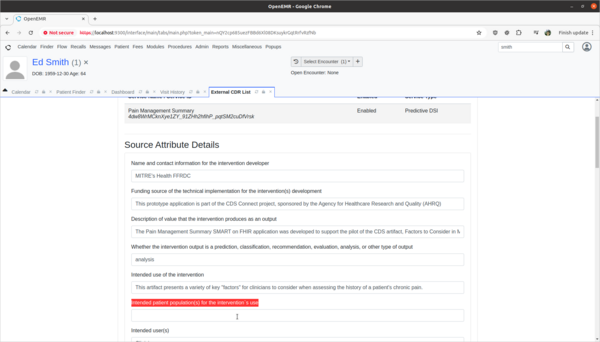
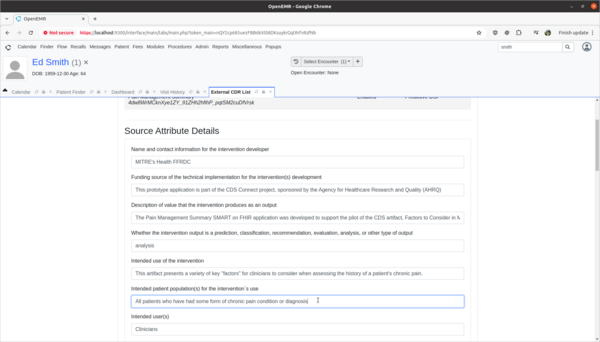
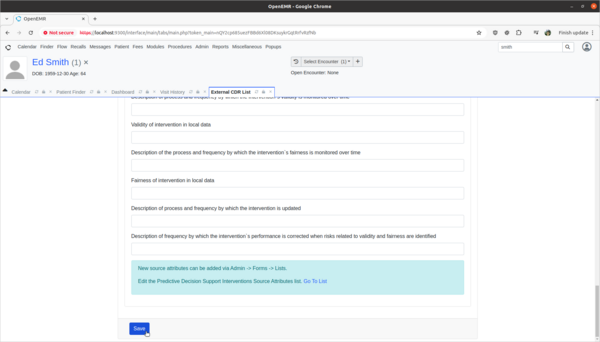
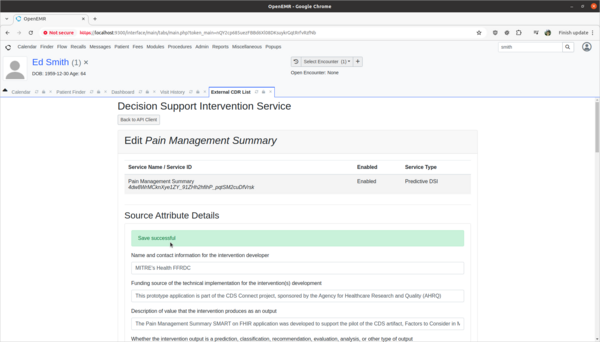
Task 3: View and update 3rd party Decision Intervention Support source attribute information
- Test:
- Update 3rd party decision support intervention source attribute field information.
- Scenario:
- The Pain Management Summary DSI engine is missing source attribute information on what kinds of patients the 3rd Party DSI will target in its AI engine. In order to provide clinicians safety information on the target populations of this DSI, this attribute needs to be updated.
- Specific Testing steps: